Metallic nature of elements in the periodic table
The study of chemistry lays on the foundation of periodic table where all the chemical miracles come from. This article presents metals, non metals and metalloids in periodic table.

Metallic nature of elements in the periodic table
Metal elements

The metals in the periodic table are the ones that hold the credit of being the first discovered elements. By the 13th century itself, ancient men used seven metals like gold, copper, silver, mercury, led, iron, tin, and hence they are known as the metals of antiquity. The majority of elements in the periodic table are metals that range from the highly reactive potassium to the non-reacting substance platinum extending in various groups. Group 1 to group 12 completely consists of metals. In the 13th group, aluminum can be seen whereas in the 14th group lead and tin remains. The Rare Earth Metals which are not easily available stand in the two lower periods. Metals are also known as electropositive elements, as they release electrons while taking part in a chemical reaction.
Non-metals
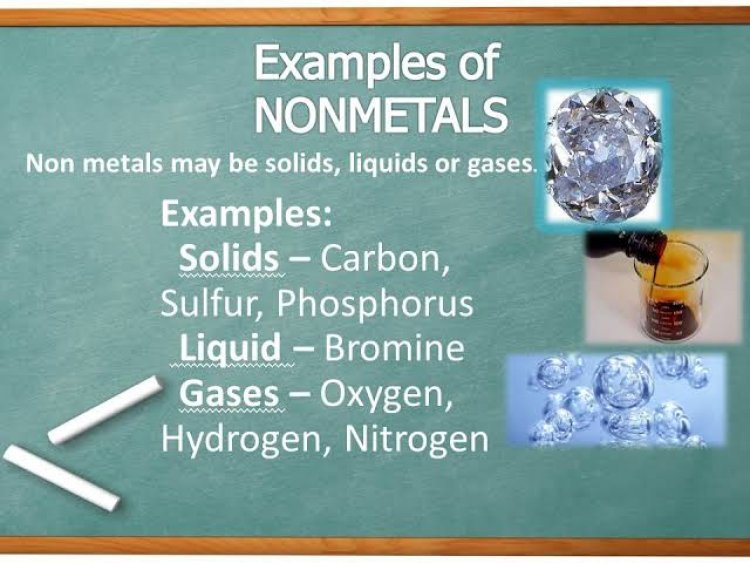
Non-metals are lesser in number even though the globe and its every aspect exist on the nonmetals. For example, 99 percent of our atmosphere is made up of two nonmetals, nitrogen and oxygen also the carbon is a vital constituent in the body of organisms. The halogen group(17th group) and the noble gas group(18th) are filled to the brim with non-metals in addition to others like hydrogen, carbon, copper, nitrogen, oxygen, phosphorus, sulfur, and selenium. Non-metals with smaller sizes are mostly seen in the gaseous states, whereas elements like carbon, phosphorus, sulfur are seen in solid-state. The only non-metal in a liquid state is Bromine. Contrary to metals, nonmetals accept or share the electrons in chemical reactions, and thus came to know as the electronegative elements.
Metalloids

Elements between the 13th and 17th groups are metalloids that look like metals but lack many metallic properties. Some scientists consider bismuth, polonium as non-metals in addition to the proper metalloids like boron, silicon, germanium, arsenic, antimony, tellurium. The contributions that metalloids granted to the electronic fields are innumerable like the formation of integrated circuits, transistors, etc. The discovery of germanium was significant as the presence of germanium was already predicted by Mendeleev. Thus, his findings became acceptable by the systematic discovery of germanium by a German scientist named Clemens Winkler in 1886. Silicon is an element that can be widely found on the earth's surface and its main source is silica or sand.
I hope, this article helped you to get a clear-cut identification and the segregation of elements in the periodic table into metals, non-metals, and metalloids.
















